7 Key Challenges in Medical Device Design and How to Solve Them

7 Key Challenges in Medical Device Design and How to Solve Them
Author: Jari Inget, Director, Connectivity Solutions, Bittium
The landscape of medical device development is shifting rapidly. The medical device sector has established itself as an integral part of the health care industry, as advanced medical device technologies have proven to deliver tangible benefits such as enabling vital sign monitoring, reduced patient recovery time, and new services. With opportunities for both the hospital and homecare sector, the medical device market’s future looks very promising. It is expected to reach an estimated $432.6 billion by 2025[1]. Thanks to advancements in science and technology, medical device manufacturers today have more opportunities than ever. At the same time, there are also new challenges. Companies need to account for several factors from rising material costs to quality control issues, growing competition, and the need for an accelerated time-to-market and new compliance demands.
The new EU Medical Device Regulations (MDR) will come into full effect in May 2021[2]. According to the regulations, manufacturers of new devices must comply with strict requirements to get their products certified. While many media reports currently cover the rise of wireless devices, the MDR makes it necessary to take one step back and look at the more fundamental challenges concerning device materials, mechanical construction, safety demands, or life-cycle support.
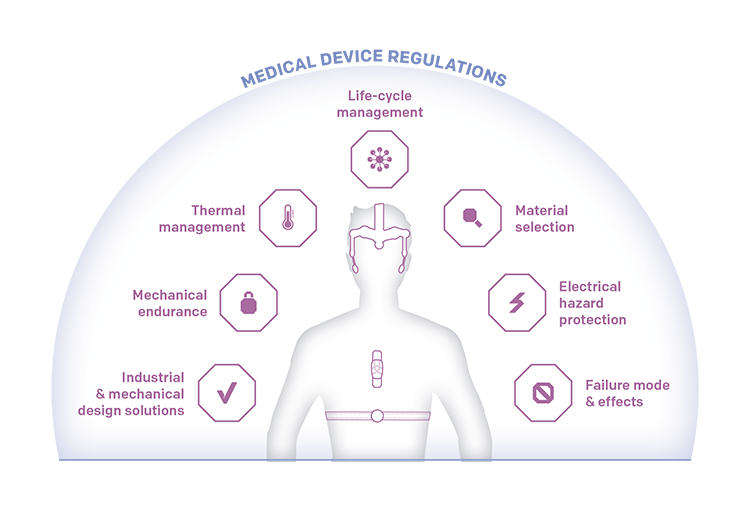
Key Challenges
Suppose we use the example of a typical mobile patient monitoring device aimed at monitoring a patient’s vital signs such as body temperature, blood pressure, ECG, oxygen saturation, or respiratory rate via sensors. In that case, these are the typical challenges to be considered:
Thermal management
Medical devices are subject to strict safety standards and usability guidelines. One essential consideration is thermal management, in which the IEC60601-1 standard regulates the surface temperature for medical devices. For devices in contact with the patient’s skin, this entails that the device’s surface temperature must stay below a specific temperature, which depends on the exact device application. Thermal simulations are an integral part of the design process. For example, simulations can help identify “hotspots” and the device construction might need changing to distribute the heat more evenly or modify the material for individual components.
In today’s battle for quick time-to-market to beat the competition, advanced simulation capabilities provide a considerable advantage as they can be done virtually without the need for early-stage prototypes. This way, it is possible to try different materials and construction set-ups within a short time frame to see how they affect the surface temperatures.
Material selection
Material selection is typically among the early steps when designing the device. Medical devices often require the use of non-allergenic materials, which are also very robust and have high endurance to sustain continuous cleaning or contact with chemicals. Also, the material might need to be RF transparent to transmit radio frequency (RF) signals. The easiest solution to making sure the material choice fulfills all compliance requirements is to use pre-certified materials for medical usage. Working with an R&D team with a comprehensive material database and simulation capabilities as described above, can streamline the material selection process considerably.
Mechanical endurance
The long service life required of many medical devices poses challenges for their mechanical endurance. Problems caused by poor mechanics quality (the design and/or manufacturing process), coupled with contact to chemicals in daily use, can lead to breakages or fractures on the device. A carefully considered design, competent manufacturers, and thorough testing can prevent such problems. In addition to the visually appealing design, quality assurance and assessment are essential.
Protection against electrical hazard
Accessible parts of the device must be protected against leakage current as regulated by the IEC60601-1 standard. It contains special requirements for medical equipment, which constitute a significant deviation from consumer products. If a risk of leakage current gets identified during the design process, accessible conductive parts of the hardware need to be exchanged or secured with additional components to prevent this from happening. Also, medical devices require a measuring function for leakage current and a control mechanism so that the device will switch off automatically if a leakage current occurs or exceeds certain limits.
Industrial and mechanics design solutions
It is very specific to medical devices that they need to be easy to clean without the danger of leaving any residue as the same devices are usually used for several patients. Thorough cleaning between the change of patients is required, and this adds another guideline for the design. The devices must fulfill IP classification because they are regularly cleaned with anti-bacterial liquids. Easy cleaning also requires a design without hard to reach nooks and clefts in which residue could build up. Finite Element Method (FEM) calculations, drop test, ingress protection, and light guide simulations make the mechanics design process more manageable. If these are included at an early stage of the design process to proof the design, the desired quality level can be reached faster, paving the way for cost savings.
Failure mode and effects
A medical device must remain safe to the user even in “single fault conditions” (SFCs) as specified in the IEC60601-1. This means that in the event of a single abnormal external condition or the failure of a single means of protection against a hazard, no safety hazard should arise for the patient. Such situations include the interruption of the protective earth conductor or one supply conductor, the appearance of an external voltage on an applied part, the failure of basic insulation, or temperature limiting devices. Due to the strict regulations, running a “failure mode and effects analysis” (FMEA) is essential in the device design process.
Life cycle management
In some cases, medical devices’ life cycle can be more than ten times longer than for consumer devices. However, nowadays, for some design areas, the same components or technologies are used in both categories, which poses challenges for medical device design. In practice, the most critical components need to be selected with the requirement in mind that the components’ availability is to be secured for the product’s entire life cycle. Systematic life cycle management includes a comprehensive second source policy for all components, managed together with all involved parties from engineering to component suppliers.
Intelligently designed medical devices play an important part in improving productivity and efficiency in healthcare and the quality of the patient care itself. At the same time, device developers and manufacturers face fierce competition from both the traditional players and new market entrants from the consumer devices sector. Implementing an efficient and high-quality R&D process to comply with the strict regulatory demands and realizing a rapid time-to-market can be the decisive factor for a medical device’s success today.
Learn more about our engineering services for Medical & Healthcare markets or contact us.
References
[1] Medical Device Market Report: Trends, Forecast and Competitive Analysis
[2] Medical Device Regulation (EU) 2017/745
Jari Inget, Director, Connectivity Solutions, Bittium
Jari Inget has over 20 years of professional experience in connectivity technologies and embedded systems development for various industries. His undying curiosity for both technology and management has led to numerous roles in both design and management while always working close to customers. He is currently responsible for business and offering development in Connectivity Solutions product and service area at Bittium.
